Before exploring chemistry, please fill out this short survey: https://forms.gle/ZUMbDD2pqfNdUrvX6
Chemistry is the study of the matter and its properties. Major subfields of chemistry include organic chemistry, analytical chemistry, physical chemistry, inorganic chemistry, and biochemistry. Organic chemistry is the study of molecules containing Carbon. Analytical chemistry is the study of molecules in a mixture. Physical chemistry is the study of how physics affects the fundamental laws of chemistry, such as how atoms behave. Inorganic chemistry is the study of molecules not containing Carbon. Biochemistry is the study of the chemical systems that make up biological processes, such as respiration. There are several career paths you can pursue with a degree in chemistry:
- Chemistry Teacher
- Forensic Scientist
- Materials Scientist
- Pharmacologist
- Toxicologist
- Biotechnologist
- Food Technologist
- Nanotechnologist
Meet Uma Chowdhry: A Materials Scientist

Uma Chowdhry was born in Bombay (now Mumbai), India in 1947. In high school, she became interested in math and science. She went on to study physics at the University of Bombay and earned her Bachelor’s in Science degree. With goals of one day becoming a world renowned scientist, she dreamed of going to a top American University and applied to several schools. She was accepted into the California Institute of Technology, or CalTech, where she planned on studying nuclear physics. However, she ended up falling in love with chemistry, particularly in the subfield of materials science. Materials science examines the structure and properties of a solid and how it is made.

In 1970, Chowdhry earned a Master’s in Engineering and then worked at Ford Motor Company. In 1972, she began studying at the Massachusetts Institute of Technology (MIT) to earn a PhD in materials science. After graduating from MIT, she began working at DuPont, one of the largest chemical companies in the United States. At DuPont, she studied ceramics, materials such as clay and porcelain, and how they could be used as superconductors. Ceramics are usually insulators, meaning they do not conduct electricity, but Chowdhry created ceramics that were superconductors, materials that have very little resistance to electric flow. She also worked on several other projects before being promoted to management at DuPont. During her time in management, she worked on creating a type of polymer used in seat cushions, paint, and other everyday items. She was elected to the National Academy of Engineering in 1996 and the American Academy of Arts and Sciences in 2003. In 2006, Chowdry became DuPont’s chief science and technology officer before retiring in 2010.
Source: https://www.sciencehistory.org/historical-profile/uma-chowdhry
Explore how the elements of the Periodic Table interact and form compounds!
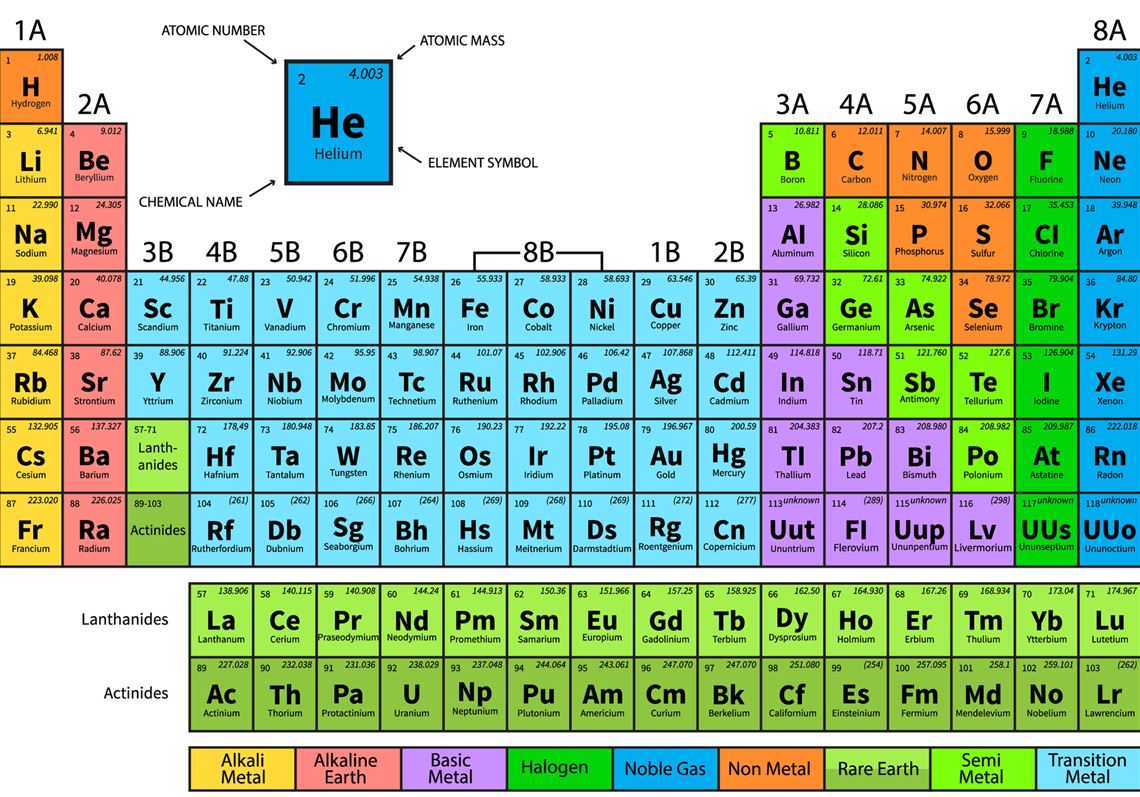
The periodic table is organized by the size of an atom of each element. There are 18 columns (sometimes referred to as groups) and 7 rows on the periodic table. Elements with more protons are bigger, so the number of protons increases as you move right across the periodic table. Each new row represents an additional outer shell of electrons.
There are 118 elements, but not all of the elements naturally occur or are stable. Uranium (element 92) is the last stable element to naturally occur on Earth. 94 elements naturally occur on Earth.
The periodic table is organized into several groups of elements. Important groups of elements include the alkali metals (column 1), alkaline earth metals (column 2), transition metals (columns 3-12), metalloids, halogens (column 17), and noble gases (column 18). The most reactive elements are the alkali metals and halogens.
Each element has information about the mass and the number of protons and electrons one atom of that element has. The atomic number represents the number of protons an element has. In a neutral atom, or an atom without a positive or negative charge, the number of electrons is the same as the number of protons. The atomic mass is found beneath the symbol and can usually be used to know how many neutrons are in an atom by subtracting the number of protons (atomic number) from the atomic mass.
goREACT is an interactive periodic table created by the Museum of Science and Industry in Chicago. Drag elements into the space below the periodic table to see which elements react with each other and which compounds they make.
https://www.msichicago.org/play/goreact/
Interested in a career in Chemistry? Here are some high school classes and extracurricular activities you can become involved in:
High School Classes:
If possible, remember to take higher level classes such as AP, IB, or honors!
- Algebra
- Geometry
- Trigonometry
- Calculus
- Physics
- Chemistry
Extracurricular activities:
- ChemClubs
- Chemistry Olympiad
- Science Olympiad
- Other Science Competitions
- Internships
- Research
- Job Shadowing
Please fill out this survey now that you have learned more about chemistry: https://forms.gle/R4EeRn7LbWxPFFsC6
Here are some more resources to help you continue your exploration of chemistry:
Additional Activities:
- Baking Soda and Vinegar Volcano (https://www.uaf.edu/museum/education/kids-families/hands-on-programs/virtualearlyexplorers/pdfs/Volcano-Experiment-Activity.pdf)
- Penny Experiments (https://sciencewithkids.com/Experiments/Chemistry-experiments/copper-plating-experiment.html)
- Red Cabbage pH Indicator (https://www.stevespanglerscience.com/lab/experiments/red-cabbage-chemistry/)
Check out these YouTube Channels
- SciShow (https://www.youtube.com/user/scishow)
- CrashCourse Chemistry (https://www.youtube.com/playlist?list=PLG61LF8I_OXoh2mhx2YNY9s4ekXiriMAf)
- Up and Atom (https://www.youtube.com/channel/UCSIvk78tK2TiviLQn4fSHaw)
- NileRed (https://www.youtube.com/user/TheRedNile)